INFLUENCE OF HORMONES ON CELLULITE: WITH EMPHASIS ON ADIPONECTIN

Content extracted from the book “Victory Against Cellulite” by Dr Roberto Chacur, Ed. AGE, 2023.
Dr. Gabriela Camargo
Dr. Roberto Chacur
INTRODUCTION
Cellulite – also known as gynoid lipodystrophy and gelatinous fibroedema – is considered a structural, inflammatory and biochemical disorder of the subcutaneous tissue, which leads to significant changes in the appearance of the skin. This occurs through chronic vascular inflammation and low-grade disturbance of the microvascular and lymphatic circulation of the subcutaneous adipose tissue (ATAMOROS et al., 2018). As for the etiology, there is a genetic-constitutional factor influenced by hormonal changes, eating habits and a sedentary lifestyle (RIVITTI, 2018).
Regarding the pathogenesis of cellulite, inflammatory factors have been associated and chronic inflammation may play an important role in the development of fibrous septa. In women, the fibrous septum is thin, with perpendicular projection; in men, however, this septum is thicker, with an oblique projection. Apparently, these histological characteristics favor the direction of expansion of the fat tissue when increased, being, in women, towards the surface and in men, towards depth. In women, the tissue is thicker; connective tissue is looser, producing larger bumps; and the fat cells are bigger. The predominance of cellulite in the female public can be explained by the difference in the organization of adipose tissue between genders (DAVID et al., 2011; BORGES; SCORZA, 2016; FRIEDMANN; VICK; MISHRA, 2017). Significant decreases in the subcutaneous expression of adiponectin (APN) – an adipocyte-derived hormone with anti-inflammatory, anti- fibrotic and vasodilatory functions may also play an important role in the pathogenesis of cellulite. Adiponectin is a glycoprotein expressed almost exclusively in adipose tissue (FRIEDMANN; VICK; MISHRA, 2017).
See Chapters
CHAPTER 1 - DEFINITION, HISTORY AND NOMENCLATURE
CHAPTER 2 - ONLINE QUESTIONNAIRE FOR CELLULITE CLASSIFICATION
CHAPTER 3 - LIPEDEMA: DESCRIPTION, DIAGNOSIS AND TREATMENT
CHAPTER 4 - ANATOMY OF THE GLUTE REGION APPLIED IN PRACTICE
CHAPTER 6 - INJECTABLE CELLULITE TREATMENTS
CHAPTER 7 - LASER-LIPO: INVASIVE TECHNOLOGY
CHAPTER 8 - OTHER CELLULITE TREATMENTS
CHAPTER 9 - BIOSTIMULATORY EFFECTS OF MICROSPHERE INJECTIONS INTO OVERLYING SKIN STRUCTURES
CHAPTER 10 - INFLUENCE OF HORMONES ON CELLULITE: WITH EMPHASIS ON ADIPONECTIN
CHAPTER 11 - GOLDINCISION®: A MULTIFACTORIAL APPROACH TO THE TREATMENT OF CELLULITE
CHAPTER 12 - STAINS POST-GOLDINCISION®
CHAPTER 13 - ADVERSE EFFECTS AND COMPLICATIONS IN GOLDINCISION®
With the collaboration of experienced medical professionals, Dr. Roberto Chacur brings together in this book an approach around the theme ranging from the genesis of cellulite, the proper method of evaluating and classifying, associated diseases and hormonal modulation to existing treatments, what really works and why the GOLDINCISION method is considered the gold standard.
CELLULITE
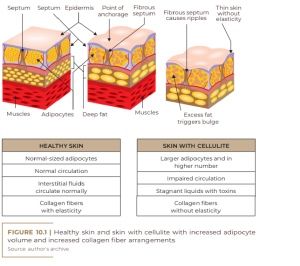
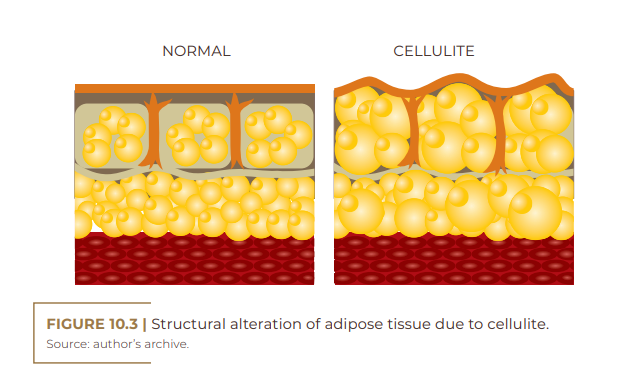
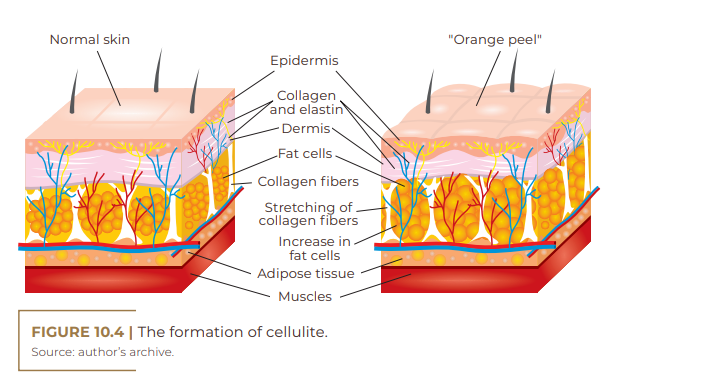
Gynoid or gynecoid lipodystrophy, a term that originates from the Greek root gynec-oid, also known as gelatinous fibroedema and cellulite, is an adipose tissue disorder more common among women, especially after adolescence. It is characterized by being a non-inflammatory process, which leads to the occurrence of irregular retraction of the skin surface; accumulation of fat (adipose tissue); ripples on the skin (orange peel- like appearance); damaged collagen fibers (dermal matrix impairment); impairment of microcirculation (fluid retention); and a knotty touch, perhaps even painful upon palpation (Figure 10.1) (RIVITTI, 2018). There are many theories about the pathophysiology of cellulite because it is complex, requiring extensive research to elucidate it. This disorder is probably multifactorial and has microcirculation failure as causes; anatomical changes; reduction in the production of the hormone adiponectin by the subcutaneous skin cell; changes in the dermal connective tissue; genetic polymorphism; and inflammatory processes (SCHONVVETTER et al., 2014; TOKARSKA et al., 2018). Multiple factors are also involved in the etiology of cellulite, including ethnic origin, gender, genetics, body biotype, distribution of adipose tissue and receptors involved, with hyperestrogenism being considered the main trigger (MACHADO et al., 2009).
Adipose tissue releases a beneficial adipokine with anti-inflammatory action – adiponectin – with the ability to synthesize and secrete leptin, which is responsible for our body’s feeling of satiety (KOKKINOFTA et al., 2012). Adiponectin also improves insulin sensitivity, suppressing the effect of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) and interferon γ (INF-γ), acting to protect against metabolic and cardiovascular diseases, which can lead to the occurrence of cellulite (COIMBRA et al., 2014).
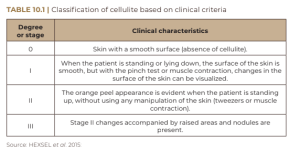
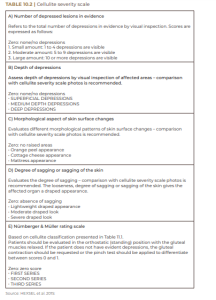
In most cases, the evaluation of cellulite is performed with physical examination, with the patient standing and muscles relaxed, so that depressed and elevated lesions are more easily identified, regardless of the pinch test or muscle contraction (HEXSEL et al., 2015). Cellulite can be classified into grades 0 through III based on clinical criteria – ranging from complete absence of cellulite to its most severe grade (Table 10.1). The classification in Table 10.1, although practical, does not cover all the important morphological aspects of cellulite. In this sense, Hexsel et al. (2009) and Hexsel et al. (2010) published the Cellulite Severity Scale, as shown in Table 10.2, based on five important clinical and morpho- logical aspects of cellulite:
A) number of depressed lesions in evidence;
B) depth of depressions;
C) morphological aspect of skin surface changes;
D) degree of sagging or sagging of the skin; E) degree of cellulite.
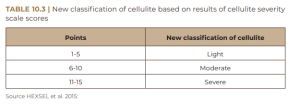
Each of these items is rated from zero to three; the sum total of their scores indicates the degree of cellulite as mild, moderate or severe, as shown in Table 10.2.
Some studies have used the cellulite severity scale to assess improvement in the condition. This is because this scale is considered objective and reliable in evaluating the results of clinical trials (HEXSEL et al., 2011; HEXSEL et al., 2013; HEXSEL et al., 2015). Regardless of the degree of cellulite, the female population is extremely affected, with a higher incidence between the ages of 15 and 45 years – the reproductive phase. This condition affects an average of 95% of post-pubertal women, of all races, with a higher prevalence in Caucasians (AFONSO et al., 2010; CUNHA; CUNHA; MACHADO, 2015). Commonly, the regions most affected by cellulite are: thighs, gluteal region and abdomen and, exceptionally, breasts, chest and arms. The regions most predisposed to increased microedema in the subcutaneous fat layers are the thighs and buttocks, due to low vascular circulation, which promotes skin abnormalities (ATAMOROS et al., 2018).
HORMONES IN CELLULITE
Considering that cellulite occurs in almost 100% of post-pubertal women, its occurrence being rare in men without androgen deficiency, female hormones certainly play a fundamental role in its etiopathogenesis (AFONSO et al., 2010; HEXSEL et al., 2012; JESUS et al., 2020). In women, estrogens are responsible for a greater number of fat cells stored in adipose tissue. The connective tissue is found radially or perpendicularly to the surface of the skin, leading to the formation of rectangular compartments that facilitate the extrusion of the papillae in the dermis-hypodermis region. This region also has larger lobes and parallel septa. In men, the fibrous septa are smaller and arranged in oblique planes with small lobes of fat (Figure 10.2). This difference explains the fact that only 2%, on average, of men develop cellulite (CRUZ et al., 2015; ARRUDA et al., 2016; MOURA; FEITOSA, 2019; TORTORA, 2019).
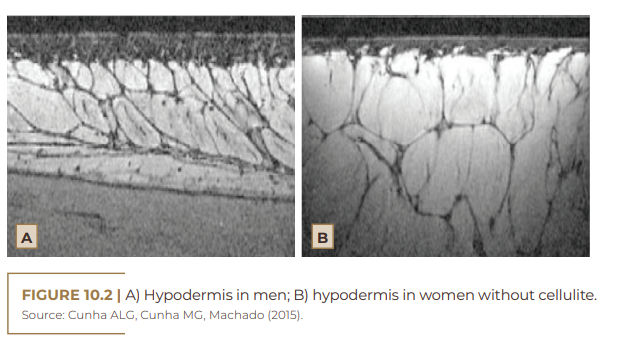
Cases of cellulite may occur in men if they have androgenetic deficiency – hypogonadism, Klinefelter syndrome, post-castration states and in patients who have used estrogen therapy for prostate cancer. The condition may worsen according to the severity of the disability (AFONSO et al., 2010). The fact that cellulite affects women almost exclusively, due to the anatomical characteristics of the hypodermis and, mainly, to the hormonal influence, has merited special attention (DAVID; PAULA; SCHNEIDER, 2011). Estrogen, among the hormones involved in the cellulite process, is considered the main development factor, in addition to being responsible for the worsening of the condition, acting at the level of:
1) amorphous fundamental substance: leads to changes in collagen and glycosaminoglycans, resulting in interstitial edema due to the accumulation of water, which leads to the fibrosclerosis characteristic of cellulite;
2) adipocytes: increases the response of α-antilipolytic receptors, stimulating the enzyme responsible for lipogenesis (LPL);
3) microcirculation: leads to a decrease in venous tonus and vasodilation (CHORILLI et al., 2007). In the pathological mechanism of cellulite, the extracellular matrix – formed by collagen and elastin fibers, as well as by amorphous interstitial material, can undergo modifications when there is an imbalance in its functioning, both by compression of its elements and by distension, which would lead to a response intended to balance the system. This matrix may have already been altered by some individual genetic factors, such as age, influence of hormones, among others (DAVID et al., 2011).
Hormonal changes can lead to dysfunctions in metabolism that can create or worsen cellulite. By way of example, in the menopause period, low estrogen production is responsible for increased vascular permeability and decreased tonus, which compromise microcirculation and are therefore important predisposing factors for the development of cellulite (LESZKO, 2014). Other aspects that contribute to the occurrence of cellulite are the effects of estrogen deficiency on the connective tissue of the skin, which include a decrease in the production and topical content of collagen and elastin fibers types I and III (LESZKO, 2014).
Estrogen is a group of steroid hormones with 18 carbons secreted mainly by the ovaries, and by the adrenal glands, but in smaller quantities. Estrogen comprises three structurally similar steroid hormones: 17β-estradiol (E2); estrone (E1) and estriol (E3). Of these, 17β-estradiol is the main steroid in humans that has estrogenic properties (KENDALL; ESRON, 2002). The relationship of hormones in women with the occurrence of cellulite is clear, when one considers that estrogen is a significant cause of its appearance, in which several factors can explain its collaboration in the etiology of cellulite. The occurrence of this is due to the change of the fatty tissue, the connectives and the vessels. In vessels, estrogen can increase or decrease irrigation in the region, compromising the tissues, which become more fibrous (MEYER et al., 2005; MENDONÇA; RODRIGUES, 2011).
The decrease in leptin receptors in the hypothalamus may be related to estrogen deficiency, which could lead to decreased satiety, increased intake, resulting in body mass gain. Estrogen decreases body weight, reducing the serum level of leptin and inhibiting food intake (THOMPSON JÚNIOR; SIITERI, 1974; SIMPSON et al., 2002). In addition, this hormone also determines the amount and disposition of body fat. During the menstrual period, there are physiological variations that can lead to edema, generating an imbalance in the integumentary system. This hormonal imbalance can culminate in the occurrence of cellulite or worsen it, especially in the reproductive phase (ELLERVICK, 2021).
The hypothalamic-adenohypophysis axis regulates the secretion of gonadal hormones. The anterior pituitary secretes folliclestimulating hormone (FSH) and luteinizing hormone (LH) in response to gonadotropin-releasing hormone (GnRH). The role of FSH in women is to regulate follicular growth and the increased production of estradiol by the granulosa cells. LH, on the other hand, is responsible for increasing cholesterol uptake, in addition to stimulating the interstitial cells of the theca of ovarian follicles to secrete androstenedione and testosterone (androgens). FSH and, mainly, LH, after ovulation, act on the luteinized cells of the granulosa and theca of the corpus luteum, leading to increased production of estradiol and, in greater quantity, of progesterone. Gonadal hormones, in this phase, regulate the secretion of GnRH, FSH and LH through a negative feedback mechanism. FSH secretion is negatively modulated by peptides produced in the ovary, such as activin and inhibin (BULUN; ADASHI, 2003). Fluid retention in women is linked to estrogen. Their bodies are programmed to store fat for use during pregnancy and breastfeeding; apparently, hormonal activity, which in certain phases of female life can excessively increase estrogen levels, is a powerful stimulant in the formation of cellulite. In the most affected regions, the lymphatic and circulatory systems are not able to oxygenate and nourish the tissues, nor to drain toxins. Based on this principle, any factor that favors fluid retention aggravates cellulite. The connective tissue becomes misshapen, evidencing this condition, because it is poorly oxygenated, undernourished and without elasticity (ZIMMERMANN, 2014).
The alterations caused by fibroblasts (mainly estrogen) in cellulite lead to the occurrence of structural changes in glycosaminoglycans (GAGs) with hyperpolymerization, leading to an increase in their hydrophilic power and to interstitial osmotic pressure, which generates the accumulation of fluid between adipocytes, resulting in collagen deposition in the interstitial matrix. The lack of uniformity in the deposition of these collagen fibers promotes irregular sclerosis of various sizes, both in blood vessels and in adipocytes. Often, changes in the size of the capillaries lead to the formation of microaneurysms, due to strangulation, causing extravasation of plasma into the interstitium, together with some cytokines and lymphocytes, reinforcing this disorder (SANT’ANA et al., 2007).
Lipolysis is an event controlled by hormones – glucagon, catecholamines, parathyroid hormone, melanocyte-stimulating hormone, thyrotropin and adenocorticotropin – in addition to adipokines and cytokines (ZECHNER et al., 2009). Adipocytes have β-adrenergic receptors (agonists) and α2-adrenergic receptors (antagonists) associated with stimulatory and inhibitory G protein, respectively (RIBEIRO, 2010). When the β-adrenergic receptor is stimulated, the membrane enzyme adenylcyclase is activated, which transforms ATP into cAMP; inactive protein kinase is responsible for lipogenesis. In the interstitial matrix, estrogen stimulates the production of fibroblasts and alters the turnover of macromolecules, leading to hyperpolymerization of hyaluronic acid and loss of elasticity of collagen fibers. In microcirculation, permeability is increased and vascular tonus is decreased, facilitating edema and decreasing blood flow, which also stimulates lipogenesis (KEDE; SABATOVICH, 2009). In the metabolism of adipose tissue there is interference of the sympathetic and parasympathetic nervous systems. Sympathetic activation stimulates lipolysis, mediated by β-adrenergic receptors, which lead to the action of the enzyme hormone sensitive lipase (HSL). Parasympathetic activation has anabolic effects, such as the uptake of glucose and fatty acids, stimulated by insulin (FONSECA-ALANIZ et al., 2006; BORGES, 2010). Other hormones such as: prolactin may also play an important role in the development of cellulite; thyroid hormones; insulin; catecholamines, including noradrenaline and adrenaline; in addition to cytokines (ELLERVICK, 2021).
FUNCTION AND STRUCTURE OF ADIPONECTIN AND ITS INFLUENCE ON CELLULITE
Adiponectin is an anti-inflammatory glycoprotein secreted in abundance in adipose tissue almost exclusively, originating from adipocytes, especially subcutaneous ones. However, it can also be secreted by the endothelium, with a vasoprotective function. Its plasma concentration acts benefiting insulin sensitivity, acting as an anti-inflammatory. The plasma concentration of adiponectin in obese individuals or those with metabolic syndrome is reduced (FRÜHBECK et al., 2017; KATSIKI, 2017). Adiponectin has a unique domain that resembles collagen, with an adherent property to nectin, giving rise to its name. It has a primary sequence of 244 amino acids, with an amino-terminal collagen domain and a carboxy-terminal globular domain, which allow the formation of multimers composed of oligomeric isoforms (MAEDA et al., 2020; MARTINEZ-HUENCHULLAN et al., 2020).
The molecular weight of adiponectin is 30 kDa. This substance is encoded by the ADIPOQ gene and is structured in 3 exons on chromosome 3q27, which has been identified as a region carrying a susceptibility gene for type 2 diabetes and metabolic syndrome. Its main circulating forms are hexameters and multimers. The three adiponectin oligomeric complexes secreted into the circulation are low (trimer – LMW), medium (hexameter – MMW) and high molecular weight (HMW). Depending on gender, plasma concentrations in this way may vary, with 5.5 µg/mL in men and 8.7 µg/mL in women (YADAV et al., 2013; TORRE-VILLALVAZO et al., 2018; MAEDA et al., 2020).
Some authors consider that adiponectin binds to three receptors: AdipoR1, AdipoR2 and T-cadherin (cell adhesion molecule that has a GPI anchor with transmembrane and intracellular domain). Despite the importance of T-cadherin, the molecular details of this receptor’s interactions with adiponectin are not fully known, since they receive less attention than the proposed interactions with AdipoR1 and AdipoR2 (PASCOLUTTI et al., 2020).
The function of adiponectin is to regulate the oxidation of fatty acids, which results in positive effects on homeostasis of energy metabolism, in addition to stimulating cytokines that act in physiological and pathophysiological processes, inhibiting pro-inflammatory cytokines, such as TNF-α and IL- 6. Adiponectin also contains properties of stimulating IL-10, which act to protect cells from apoptosis, induced by inflammatory cytokines (YANAI; YOSHIDA, 2019; DI ZAZZO et al., 2019). The realization of the cytokine induction and inhibition mechanism occurs through the signaling of its receptors AdipoR1 (this is highly expressed in skeletal muscle) and AdipoR2 (which is more restricted to the liver). The discovery of these receptors occurred from cells transfected with a complementary DNA (cDNA) of human skeletal muscle, using a recombinant agent in the globular form of adiponectin (FANG; JUDD, 2018; YANAI; YOSHIDA, 2019; MAEDA et al, 2020).
Emanuele et al. (2011) believe in the hypothesis that adiponectin expressed in subcutaneous adipose tissue (SAT) may play a role in the pathogenesis of cellulite. In this sense, they compared the levels of gene expression of adiponectin in the TAS taken from cellulite in the gluteal region with the levels in the TAS taken from the same region in women without cellulite. Plasma levels of adiponectin were also measured in women with and without cellulite. The study included 30 women, 15 thin with cellulite (BMI < 25 kg/m 2) and 15 without cellulite matched for age and BMI. Real-time reverse transcription polymerase chain reaction (RT-PCR) was used to assess adiponectin.
Adiponectin messenger RNA (mRNA) expression in the gluteal SAD was significantly lower in areas with cellulite compared to those without. However, plasma levels of adiponectin did not differ between women with and without the manifestation of this condition. It can be concluded that adiponectin expression is significantly reduced in SAD in areas affected by cellulite.
CONCLUSION
Cellulite is a complex, multifactorial disorder of the subcutaneous fat layer and the overlying superficial skin. On the other hand, adiponectin, a hormone derived from adipocyte produced by subcutaneous fat, has important protective anti-inflammatory and vasodilator effects, and may play a key role in the pathogenesis of cellulite. It is believed that cellulite, being multifactorial, can be caused, among other factors, by the reduction in the production of adiponectin by the subcutaneous tissue.
We commonly receive similar patients, many of whom are being monitored by nutritionists and are using supplements that help with body definition, with fat loss. There is general satisfaction with the body, thighs and abdomen, however, as the female gluteal region has a large percentage of fat, its size ends up decreasing drastically when the percentage of fat is reduced (although the appearance of cellulite may improve), in a level greater than the volumetric gain of the gluteus maximus, medius and other muscles, and this ends up causing even greater dissatisfaction in this region, leading many nutrologist colleagues to indicate to patients the replacement of the lost volume with fillers, since these patients would also not have the possibility of carrying out treatments with liposculpture, due to the presence of too few fat for fat grafting. In the case above, intramuscular volumetry was associated, with a total of 200 ml of Biosimetric® 30% on each side, in two steps, associated with the Goldincision® method, improving the general quality of the skin, restructuring the collagen, and implementing the circulation and the local metabolism.
REFERENCES
Afonso JPJM et al. Celulite: artigo de revisão. Surg Cosmet Dermatol. v. 2, n. 3, p.214-19, 2010.
Arruda EF et al. Recursos fisioterapêuticos utilizados no tratamento do fibro edema geloide (FEG). Revista Científica da Faculdade de Educação e Meio Ambiente. v. 7, n. 2, p.45-58, 2016.
Atamoros FMP et al. Evidence-based treatment for gynoid lipodystrophy: A review of the recent literature. J Cosmet Dermatol. v. 17, p.977-983, 2018.
Borges FS. Dermato-funcional: modalidades terapêuticas nas disfunções estéticas. 2.ed. São Paulo: Phorte, 2010. Borges FS, Scorza FA. Terapêutica em estética: conceitos e técnicas. São Paulo: Phorte, 2016.
Bulun SE, Adashi EY. The physiology and pathology of the female reproductive axis. In: WILSON, J.D.; FOSTER, D.W. Williams Textbook of Endocrinology. 11th ed. Philadelphia: WB Saunders Company, 2003.
Chorilli M et al. Avaliação histológica da pele após exposição à gel acrescido de hialuronidase associado ou não a ultra-som. Lat. Am. J. Pharm. v. 26, n. 1, p.26-30, 2007. Coimbra S et al. Circulating levels of adiponectin, oxidized LDL and Creactive protein in Portuguese patients with psoriasis vulgaris, according to body mass index, severity and duration of the disease. J. Dermatol. Sci. v. 55, n. 3, p.202-204, 2014.
Cruz JCR, Ueno NF, Manzano BM. O estudo científico como base na área da estética: uma contrapartida ao senso comum. Revista Científica da FHO – UNIARARAS. V. 3, n. 2, p.85-93, 2015.
Cunha MG, Cunha ALG, Machado CA. Fisiopatologia da lipodistrofia ginoide. Surg Cosmet Dermatol. v. 7, n. 2, p. 98-103, 2015.
David RB, De Paula RF, Schneider AP. Lipodistrofia ginoide: conceito, etiopatogenia e manejo nutricional. Revista Brasileira Nutrição Clínica v.26, n.3, p.202-206, 2011.
Di Zazzo E et al. Adiponectin as Link Factor between Adipose Tissue and Cancer. International Journal of Molecular Sciences. v. 20, n. 4, p.839, 2019.
Ellervik C. Perspective on cellulite. Dermatology and Dermatologic Diseases. v. 8, n. 6, p.307, 2021. Emanuele E et al. Adiponectin expression in subcutaneous adipose tissue is reduced in women with cellulite. International Journal of Dermatology. v. 50, p.412-416, 2011.
Fang H & Judd RL. Adiponectin Regulation and Function. Comprehensive Physiology. v. 8, n. 3, p.1.031-1.063, 2018. Fonseca-Alaniz MH et al. O tecido adiposo como centro regulador do metabolismo. Arq Bras Endocrinol Metab. v. 50, n. 2, p.216-229, 2006.
Friedmann DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clinical, Cosmetic and Investigational Dermatology. v. 10, p.17-23, 2017.
Frühbeck G et al. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Scientific Reports. v. 7, n. 1, 2017. Hexsel D et al. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. v. 23, n. 5, p.523-8, 2009.
Hexsel D et al. Definition, clinical aspects, classifications, and diagnostic techniques. In: GOLDMAN, M.P. et al. Cellulite pathophysiology and treatment. New York: Taylor & Francis; 2010.
Hexsel D et al. A bipolar radiofrequency, infrared, vacuum and mechanical massage device for treatment of cellulite: a pilot study. J Cosmet Laser Ther. v. 13, n. 6, p.297-302, 2011.
Hexsel D et al. Avaliação dos aspectos psicológicos, psiquiátricos e comportamentais de pacientes com FEG: estudo piloto. Surg Cosmet Dermatol. v. 4, n. 2, p.131-6, 2012.
Hexsel D et al. Noninvasive treatment of cellulite utilizing an expedited treatment protocol with a dual wavelength laser-suction and massage device. J Cosmet Laser Ther. v. 15, n. 2, p.65-9, 2013.
Hexsel D et al. Cellulite: Classification and Scoring. ResearchGate. DOI: 10.1007/978-3-319-26594-0_93-1. Available at: https://www.researchgate.net/publication/ 314921499_ Cellulite_Classification_and_Scoring. Accessed on: July 17, 2022.
Jesus CBR. Estratégias terapêuticas no manejo do fibro edema gelóide. Journal of Applied Pharmaceutical Sciences.
2020. Katsiki N et al. Adiponectin, lipids and atherosclerosis. Current Opinion in Lipidology. v. 28, n. 4, p.347-354, 2017.
Kede MPV, Sabatovich O. Dermatologia Estética. 2. ed. Atheneu, 2009. Kendall B, Esron R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med. v. 32, n. 2, p.103-23, 2002.
Kokkinofta R et al. Risk factors of obesity in a cohort of 1001 Cypriot adults: an epidemiological study. Hippokratia, Thessalonike, v. 16, n. 3, p.256-260, 2012.
Leszko M. Cellulite in menopause. Prz Menopauzalny. v.13, n. 5, p.298-304, 2014.
Machado AFP et al. Incidência de fibro edema geloide em mulheres caucasianas jovens. Arq Bras Ciên Saúde. v. 34, n. 2, p.80-86, 2009.
Maeda N et al. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. v. 292, p.1-9, 2020.
Martinez-Huenchullan SF et al. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism. v. 102, p.154008, 2020.
Mendonça RSC, Rodrigues GBO. As principais alterações dermatológicas em pacientes obesos. ABCD, Arq. Bras. Cir. Dig. v. 24, n. 1, p,68-73, 2011.
Meyer PF et al. Desenvolvimento e aplicação de um protocolo de avaliação fisioterapêutica em pacientes com fibro edema geloide. Fis. em Mov. v. 18, n. 1, p.75-83, 2005.
Moura LRM, Feitosa AORM. Análise dos efeitos do ultrassom terapêutico no fibro edema geloide (celulite). Revista da FAESP. v. 3, n. 4. p.21-29, 2019
Pascolutti R et al. Mapping and engineering the interaction between adiponectin and T-cadherin. Journal of Biological Chemistry. v. 295, n. 9, p.2.749-2.759, 2020.
Rivitti E. Dermatologia de Sampaio e Rivitti. 4. ed. São Paulo: Artes Médicas, 2018. Sant’Ana EMC, Marqueti RC, Leite VL. Fibro edema geloide (celulite): fisiopatologia e tratamento com endermologia. Fis Esp. v. 1, n. 1, p.30-5, 2007.
Schonvvetter B et al. Longitudinal evaluation of manual lymphatic drainage for the treatment of gynoid lipodystrophy. Anais Brasileiros de Dermatologia. v. 89, n. 5, p.712-18, 2014.
Simpson ER et al. Aromatase – a brief overview. Annu Rev Physiol. v. 64, p.93-127, 2002.
Tassinary J. Raciocínio clínico aplicado à estética corporal. 2018. Available at: https://issuu.com/editoraesteticaexperts/docs/cap-2 blog_celulite. Accessed on: Jul 18. 2022.
Thompson Junior EA, Siiteri PK. The involvement of human placental microssomal cytochrome P-450 in aromatization. J Biol Chem. v. 249, n. 17, p.5.373-5.378, 1974.
Tokarska K et al. Cellulite: a cosmetic or systemic issue? Contemporary views on the etiopathogenesis of cellulite. Advances in Dermatology and Allergology v. 35, n. 5, p.442-446, 2018.
Torre-Villalvazo I et al. Adiponectin synthesis and secretion by subcutaneous adipose tissue is impaired during obesity by endoplasmic reticulum stress. Journal of Cellular Biochemistry. v. 119, n. 7, p.5.970-5.984, 2018.
Tortora GJ. Princípios de Anatomia Humana. Guanabara Koogan, 2019.
Yadav A et al. Role of leptin and adiponectin in insulin resistance. Clinica Chimica Acta. v. 417, p.80-84, 2013.
Yanai H, Yoshida H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. International Journal of Molecular Sciences. v. 20, n. 5, p.1.190, 2019.
Zechner R et al. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. Thematic Review. v. 50, n. 1, p.3-21, 2009. Zimmermann L. Celulite. Revista Vida Estética. v. 112, p.48-55, 2994.
