BIOSTIMULATORY EFFECTS OF MICROSPHERE INJECTIONS INTO OVERLYING SKIN STRUCTURES

Content extracted from the book “Victory Against Cellulite” by Dr Roberto Chacur, Ed. AGE, 2023.
Dr. Gottfried Lemperle
Frankfurt, Germany
MAIN TAKEAWAY
1. Biostimulation is in fact the normal reaction of a foreign body to all filler microspheres.
2. Neocollagenesis occurs only after long-lasting and permanentEllansé® PMMA microspheres.
3. PMMA implants are “living implants” that bleed when cut.
4. Sculptra® and Radiesse® are dissolved by giant cells before neo- collagenesis can begin.
5. Flattened skin structures are caused by intradermal edema of the extracellular matrix that surrounds all foreign body reactions. Edema facilitates the migration of macrophages and the exchange of molecules (MARMUR; PHELPS; GOLD- BERG, 2004).
INTRODUCTION
Over the past 10 years, nearly all dermal filler manufacturers and their injecting physicians have claimed that their products stimulate neocollagenesis after injection (Haddad et al., 2022). Stimulation of new collagen is a result of fibroblast activation. Conventional wisdom has been that the activation of fibroblasts by means of fillers resulted exclusively from the use of those fillers categorized as biostimulators, among which is poly-L- lactic acid, which produce a cutaneous inflammatory reaction.
Greater understanding of non-inflammatory fillers such as calcium hydroxyapatite has helped to clarify how non-inflammatory fillers stimulate the production of new collagen. Recent studies have proven the effectiveness of new biostimulating fillers that are entering the market, for example polycaprolactone, as well as the hypothesis that cross-linked hyaluronic acids act as a structural support for collagen-producing fibroblasts (Handler; Goldberg, 2018).
That sounds much more pleasant than “our product stimulates an inflammatory foreign body reaction”. However, the histological evidence for this claim as well as a second claim, that the product also softens superficial skin structures, is either non-existent or unconvincing. “Conventional wisdom” without evidence is not a scientific argument. The search for evidence of biostimulation and neocollagenesis boils down to a few facts. Dermal fillers can be divided into two categories: pure volumizers such as hyaluronic acids (HA) secreted by fibroblasts (Wang et al., 2007) (Figure 9.1) and polyacrylamide gel (PAAG) (Buzzaccarini et al., 2022) that are slowly degraded by secreted hyaluronidases and hydrolases and taken up again by macrophages, often without much effort from other cells (Figure 9.2). On the other hand, pure stimulators, like most particulate materials, initiate a strong cellular reaction after injection, which often replaces the carrier volume. There are two carriers for microspheres: bovine atelo-collagen in Artecoll® and Bellafill®; and carboxymethylcellulose (CMC) in Brazilian products with PMMA Biossimetric® and Linnea safe®, as well as in Sculptra® (PLLA), Radiesse® (CaHA) and Ellansé® (PCL). All are absorbed within the first few days after injection, leaving the microspheres agglomerated in the tissue.
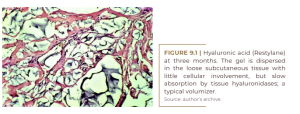
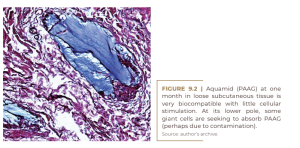
Invading monocytes and macrophages and emerging giant cells, as the first raw materials of external defense, slowly fill the spaces between the microspheres. In the subsequent weeks, fibroblasts and capillaries will complete the picture. Clinically, this exchange causes a slight indentation in the skin of the injected areas, but this is leveled out within four weeks by defense cells or ingrown connective tissue.
Collagen fibers first appear after one year, when most temporary fillers have been absorbed. Histological reactions to microsphere injections are extremely varied, according to their chemical structure: they range from a slight cellular involvement around PMMA (Biossimetric®) and calcium spheres in Radiesse® (ALMEIDA et al., 2019; Yutskovskaya; Kogan, 2017) to an expressive granulomatous reaction around the polycaprolactone microspheres in Ellansé® (Kim, 2020).
Since many desires and misinformation are described and repeated in the literature, the histological reactions to the four individual injectable stimulators are described and discussed here. These facts are causing a possible effect of injectables on overlying skin structures.
See Chapters
CHAPTER 1 - DEFINITION, HISTORY AND NOMENCLATURE
CHAPTER 2 - ONLINE QUESTIONNAIRE FOR CELLULITE CLASSIFICATION
CHAPTER 3 - LIPEDEMA: DESCRIPTION, DIAGNOSIS AND TREATMENT
CHAPTER 4 - ANATOMY OF THE GLUTE REGION APPLIED IN PRACTICE
CHAPTER 6 - INJECTABLE CELLULITE TREATMENTS
CHAPTER 7 - LASER-LIPO: INVASIVE TECHNOLOGY
CHAPTER 8 - OTHER CELLULITE TREATMENTS
CHAPTER 9 - BIOSTIMULATORY EFFECTS OF MICROSPHERE INJECTIONS INTO OVERLYING SKIN STRUCTURES
CHAPTER 10 - INFLUENCE OF HORMONES ON CELLULITE: WITH EMPHASIS ON ADIPONECTIN
CHAPTER 11 - GOLDINCISION®: A MULTIFACTORIAL APPROACH TO THE TREATMENT OF CELLULITE
CHAPTER 12 - STAINS POST-GOLDINCISION®
CHAPTER 13 - ADVERSE EFFECTS AND COMPLICATIONS IN GOLDINCISION®
With the collaboration of experienced medical professionals, Dr. Roberto Chacur brings together in this book an approach around the theme ranging from the genesis of cellulite, the proper method of evaluating and classifying, associated diseases and hormonal modulation to existing treatments, what really works and why the GOLDINCISION method is considered the gold standard.
FILLING WITH PMMA
PARTICULATE DERMAL FILLERS
Sculptra®
Poly-L-lactic acid (PLLA), a synthetic, biocompatible and biodegradable polymer, has been used safely in many clinical applications over the past decades. Sculptra® can be categorized as a stimulating filler, as it stimulates the synthesis and deposit of fibrous tissue and collagen. In most studies, the effect of Sculptra® on collagen synthesis was investigated in vivo and most of the data came from clinical and histological reports. There is only one study reporting this effect in vitro using fibroblasts. Here, we investigated whether PLLA in the form of nanoparticles can provide the same effect on collagen synthesis in fibroblasts as Sculptra®. Surprisingly, we found that there was no collagen stimulation only in fibroblasts; on the other hand, cocultures of fibroblasts and macrophages showed collagen stimulation by PLLA nanoparticles (Ray, 2009, (p.1-9).
Sculptra® was introduced in 1999 and is distributed today by Galderma Laboratories (Dallas, Texas). As one of the first filler companies, Galderma came up with the slogan of “neocollagenesis” in 2009 (LACOMBE, 2009), an acquisition of a histological fact that occurs daily in wound repair. Sculptra® can be described as a stimulator of a foreign body reaction and is just another volumizer whose effect disappears when the last microsphere is absorbed by the body (Lemperle; Morhenn; Charrier, 2020).
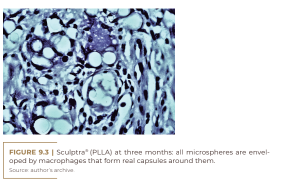
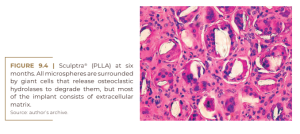
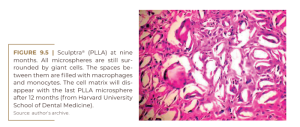
Sculptra® contains slowly absorbable microspheres of poly-L-lactic acid (150 mg/vial) to be suspended in 5, 10 or even 18 ml of carboxymethylcellulose gel, to avoid the formation of nodules in the subcutaneous tissue. The microspheres are enveloped by macrophages (Figure 9.3) which soon fuse with giant cells (Figure 9.4) and begin to decay at nine months (Figure 9.5) with no histological sign of collagen fibers. At this time, for instance, fibroblasts in PMMA implants secrete collagen fibers to hold the PMMA microspheres in place for life and form permanent connective tissue, including capillaries.
No data were found in the scientific literature or on the Internet to prove alleged neocollagenesis after PLLA injections. Rather, its bulk is populated only by giant cells, monocytes, and macrophages, but obviously few fibroblasts and collagen fibers (Figure 9.4).
Radiesse®
Radiesse® is a biocompatible, biodegradable and resorbable biostimulating filler that can stimulate endogenous collagen production. It is a unique product that provides both volume replacement and collagen biostimulation as its primary mechanism of action. After approximately 9 or even 12 months, the CaHA particles are degraded into calcium and phosphate and are eliminated by the renal system. Immediate correction is gradually followed by formation of new tissue through neocollagenesis, elastin production, angiogenesis, and cell proliferation. The result is lasting aesthetic improvement for ≥18 months, with firm and elastic skin and increased skin thickness (ALMEIDA et al., 2019). Radiesse® (Merz Aesthetics, Frankfurt, Germany) is the best biocompatible injectable of all, due to its natural component calciumhydroxyl-apatite, the molecule from which bones and teeth are made (Yutskovskaya; Kogan, 2017). Its microspheres are suspended in carboxymethylcellulose and cause the lowest side effects of all injected particulate materials, especially foreign body granulomas (Lemperle; Morhenn; Charrier, 2020).
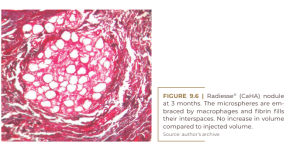
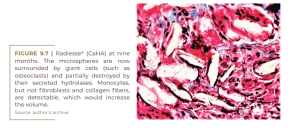
On the other hand, this product stimulates a mild foreign body reaction (Figure 9.6) and is absorbed after nine months as small bone fragments by osteoclastic hydrolases, with strong cellular involvement in a common foreign body reaction (Figure 9.7) (Lemperle, 2009).
Soon after Sculptra®, the Radiesse® community adopted Galderma’s and the Sculptra® community’s wishful thinking, in addition to also claiming biostimulation and neocollagenesis as their main secrets (ALMEIDA et al., 2019; YUTSKOVSKAYA; KOGAN, 2017).
Ellansé®
Ellansé® collagen stimulator is composed of bioabsorbable PCL microspheres suspended in an aqueous carboxymethylcellulose gel carrier. In addition to the soft tissue plumping effect, the microspheres stimulate the production of new collagen, resulting in volume restoration, remodeling and improved skin quality. Three versions are available (Ellansé-S®,-M®, -L®), providing a duration of effect of at least 18 months and up to 3 years, as the degradation time of PCL microspheres depends on the initial length of the polymeric chain. These fillers, also known as collagen stimulators, are characterized by their long duration of action and their biostimulating properties: the increase in collagen production that follows their implantation prolongs their duration of action (CHRISTEN; VERCERI, 2020).
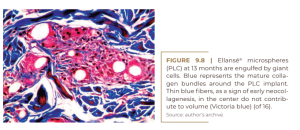
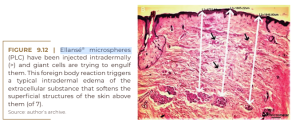
Ellansé® (Sinclair Pharma, London) is the youngest dermal filler on the market, having been introduced in 2009. Since its introduction, it has been claimed as a “collagen booster”. Ellansé® is composed of 4 polymerized microspheres of polycaprolactonic acid (PCL), 30% of which are suspended in 70% of carboxymethylcellulose (CMC). According to their different polymerizations, polyesters are slowly degraded over the years by hydrolysis of ester bonds. The neocollagenesis hypothesis (CHRISTEN; VERCERI, 2020) was confirmed by Kim in 2020; the histological images in his two previous papers, from 2015 (KIM; VAN ABEL, 2015) and 2019 (KIM, 2019), were not convincing. After 1,000 intradermal injections of LCP through an automated injector into the temporal skin of 13 women, he found a 26.7% increase in collagen fibers after four years. He also described an improvement in skin texture by 30% at six months and temporal dermis thickening by 11.1% – from 2.15 mm to 2.7 mm (KIM, 2019). Their histological images show large numbers of foreign body giant cells just after injection, which are trying to engulf each PCL microsphere (Figure 9.8), which is similarly seen only in filling granulomas (LEMPERLE et al., 2009). Only Sculptra® shows a 100% giant cell rate in its initial foreign body reaction (Figure 9.4). Giant cells are the typical sign of a foreign body granuloma when macrophages alone are not able to destroy the material (LEMPERLE et al., 2009).
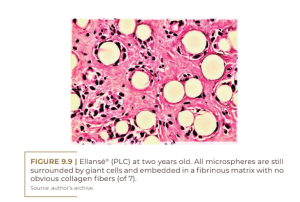
Giant cells are usually formed by the fusion of frustrated macrophages that alone cannot degrade PCL microspheres. This strong stimulation of the PCL suggests a toxic substance within the PCL itself (possibly its catalyst). In his figure 3A and 3B after 1 and 4 years, Kim describes unrecognizable new “extremely fine collagen fibers” (Figure 9.8), which cannot account for the swelling after LCP injections. The true swelling is primarily due to the significant amount of giant cells and due to the edematous extracellular matrix, filled with monocytes and macrophages (Figure 9.9).
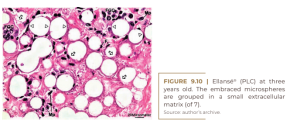
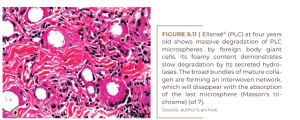
At two years, all of the microspheres are still surrounded by giant cells and embedded in the fibrinous extracellular matrix without obvious collagen fibers (Figure 9.9). At three years, there is no significant histological change, except for a clumping of the microspheres (Figure 9.10). The small size of the giant cells and their absence in the lower right corner suggests that these microspheres are located in the center of the implant, where they have not yet been reached by the giant cells. This may suggest confusion with a previous biopsy. Finally, at four years of age, we see clearly defined bundles of collagen appearing for the first time in the dermal filler literature for another PMMA filler (KIM, 2020) (Figure 9.11). It is surprising that these mature collagen fibers are not found after three years (Figure 9.10).
Increased dermal thickening after intradermal injection of PCL is demonstrated in Kim’s Figure 1B (Figure 9.12). At first glance, this is a typical dermal edema caused by toxic PCL microspheres engulfed by defense giant cells (see arrows). This usual swelling is the explanation for the smoothing effect of the superficial structures of the overlying skin.
PMMA – Microspheres
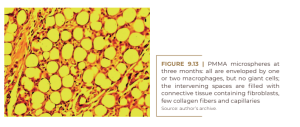
Injectable, non-absorbable PMMA microspheres of 40 μm in size were developed in the early 1980s, as a response to the short-acting Zyderm® and Zyplast® bovine collagen injections (Lemperle et al., 1991). They were introduced in Brazil in 1996 and manufactured for many years under the name Metacrill® (SERRA; GONÇALVES; RAMOS-E-SILVA, 2014; Chacur, 2019). Today, two similar injectables composed of PMMA are approved by the Brazilian National Health Surveillance Agency (ANVISA) (SOUZA et al., 2018): Biossimetric® (MTC Medical Comércio e Indústria, from Anápolis, Goiás) and Linnea Safe® (Laboratório Lebon, from Porto Alegre). Biosimetric® PMMA microspheres are uniform in size, free of small particles and their surfaces still provide sufficient roughness for macrophage attachment (Lemperle, 2022). Both differ from Artecoll-4 from China and Bellafill from the US in terms of their carrier, which is carboxymethylcellulose (CMC), commercially available in Biosimetric® or hydroxyethylcellulose (in Linnea Safe) – rather than expensive bovine collagen by Artecoll and Bellafill (Ronan et al., 2019). The three carriers are absorbed in the first few days and leave the microspheres agglomerated and fixed at the injection site. Within a week, macrophages invade the outer edge of the microspheres (Lemperle, 2022), and within 3 months, all microspheres are encapsulated with two or three macrophages. Fibroblasts advance into the extracellular matrix to produce capillaries for nourishment and renewal (Figure 9.13).
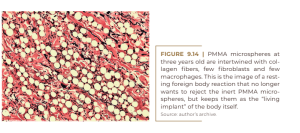
After nine months, most of the useless macrophages leave the scene or are phagocytosed after apoptosis. In this way, fibroblasts, collagen fibers, and capillaries are filling the interspaces (Figure 9.14). By age 10, all the microspheres are intertwined with fibroblasts and large collagen fibers (Figure 9.15) and the arterioles and venules have converted the injected PMMA into a “live implant” that bleeds when cut. Histologically, there is no detectable difference between the different PMMA injectables, as the four companies eliminated small PMMA particles below 20 μm, which were phagocytosed and transported to the lymph nodes, lung or liver (Lemperle; Morhenn; Charrier, 2020).
These small particles may have been the supposed cause of the late formation of the foreign body granuloma, given that their chemical structure remained in the memory of the macrophages. A patient’s systemic bacterial infection was often the trigger for a granulomatous reaction at all previously affected sites (LEMPERLE et al., 2009).

DISCUSSION
Defense mechanisms against injected foreign materials
If particles are injected into the human body, the surrounding damaged cells release cytokines into the tissue, which causes nearby monocytes to convert to macrophages. This first rank of warriors tries to phagocytose the intruding particles or render them harmless by encapsulating them with their cell bodies (Figure 9.12). Macrophages have a size that varies from 10 μm to a maximum of 60 μm, that is, they cannot march with an embraced microsphere of 40 μm in diameter. Thus, they remain linked to the enemy like Siamese twins, temporarily with absorbable microspheres in Sculptra®, Radiesse® and Ellansé® and permanently with PMMA microspheres in Artecoll, Bellafill, Biosimetric® or Linnea Safe®.
As all cells require oxygen, fixed macrophages send more cytokines and attract more macrophages and fibroblasts, which build a regular connective tissue with capillaries and small vessels, that is, they form a living implant, which bleeds when cut. However, a foreign body reaction also promotes increased blood circulation and local metabolism, with proven increase in temperature by body thermography. Neocollagenesis occurs in the human body as a natural component of wound repair, as a result of an inflammatory response to injury. A second task of fibroblasts is the wrapping and attachment of permanent foreign bodies, such as breast implants or inert microspheres. Unlike neocollagen (Figure 9.7), bundles of mature collagen can only be detected and demonstrated on histological images of Ellansé® and PMMA within the first nine months after injection (Figures 9.11 and 9.14). This is a period when half of the injected PLLA, CaHA or PCL microspheres have already been absorbed. Unfortunately, only a few histological images exist of these other three dermal injectables. Special staining with picrosirius red or Masson’s trichrome could provide evidence of claimed and possible collagen fibers.
Loss of skin elasticity and “cellulite”
About 10 years ago, some injectors of different dermal fillers noticed that the overlying skin structures improved after injecting certain products. A study by Goldberg et al. (2013) on the response of human tissue to Sculptra® “opened a new class of collagen stimulators” (CHRISTEN, 2022), which paraphrases in more enticing words a simple foreign body reaction. Cavallini et al. (2019) evaluated skin quality six months after intradermal injection of a newly developed viscous HA (VYC-12) in 40 women with a new “Digital Skin Surface Analysis” (DACS), and found a texture that improves 30%. Such an effect after intradermal injections is not uncommon and is absolutely understandable.
Kim (2019) measured dermal thickness one year after intradermal injection and found PCL microspheres surrounded by giant cells and some scattered fibroblasts. Surrounding edema with clearly spaced collagen fibers was the cause of this increase in thickness (Figure 9.12). Bravo et al.(2022) injected hyaluronic acid (HA) and Radiesse® into 15 women in a subdermal plane and measured an 11.1% increase in dermal thickness after four months. The facial dermis has an average thickness of 1 mm; 11.1% increase is 0.11 mm, the thickness of the epidermis. Excitement with this dermal biostimulation was also high. As is often the case when it comes to cosmetics, there are few facts but a lot of wishful thinking involved. There is no doubt that the skin’s elasticity decreases with age and that the extreme thinning and wrinkling that occurs in the face of some women is likely to be due primarily to later laxity of genetic origin.
Dermal neocollagenesis is often seen as the main reason for the visible improvement of the skin after different non-invasive and minimally invasive aesthetic treatments. However, the very slow dynamics of mature collagen remodeling in the extracellular matrix of the dermis, with a half-life of 15 years, makes each observable increase in collagen production insufficient to replace a significant part of the matrix during the short period in which it takes place. states that improvement of the skin occurs (KRUGLIKOV, 2013).
Furthermore, the change from type 3 collagen to type 1 collagen, which is thicker and more resistant, only takes place after nine months. Therefore, the observed effect must be attributed to the natural edema that accompanies all temporary and permanent cellular reactions. The buttocks play an important role in physical attractiveness and have always been considered a criterion of female beauty associated with fertility and health status. With aging, the skin loses its elasticity. Loss of gluteal subcutaneous fat and looseness of the interlobular septa lead to decreased volume in the area and gluteal ptosis (HEXSEL; MAZZUCO, 2000; KAMINER et al., 2019; COHEN et al., 2020; YOUNG VL, DI BERNARDO, 2021).
The gluteal suspension system, a dense ligamentous connective tissue, becomes less firm, leading to sagging of the buttocks. This process leads to a compromise in the quality of the skin, with the development of stretch marks and indentations derived from cellulite, which particularly affect the buttocks and thighs of 80% of women. This multifactorial condition is a real concern and consequently generates
increased interest in the aesthetics of this region ( SERRA; GONÇALVES; RAMOS-E-SILVA, 2014; CHACUR, 2019; HEXSEL; MAZZUCO, 2000; DAVIS; BOEN; FABI, 2019).
Adverse events from subcissions can include:
a) Formation of nodules if the PMMA or temporal filler does not spread into the surrounding tissue – which can be prevented by further diluting the PMMA to 30%.
b) Seroma formation if the small subcision spaces communicate and allow fluid to accumulate.
c) Hemosiderin and skin hyperpigmentation above nodules and seromae (HEXSEL; MAZZUCO, 2000; DAVIS; BOEN; FABI, 2019). Interestingly, granuloma formation occurred only after intradermal or high subdermal filler injections in the dermo-subdermal plane, and not after injections deeper into the subcutaneous or epiperiosteal fat in the bones, nor intramuscularly after deep muscle or buttock augmentation.
The explanation for this phenomenon is the fact that the dermis is the most sensitive and immunologically active organ. This fact is important when it comes to subcision ( HEXSEL; MAZZUCO, 2000 ) of fibrous bands under skin dimples (cellulite) and immediate filling with particulate materials ( DAVIS; BOEN; FABI, 2019 ). Particulate injectables can improve skin structures. Every injectable is initially a foreign body that disturbs tissue integrity. Surrounding fibroblasts or damaged cells release fear cytokines, which cause swelling and attract macrophages to help defend against foreign bodies. If we look at subcutaneous loose connective tissue, the spaces between individual collagen fibers vary from about 5 μm to 10 μm in width, which is the width of a fibroblast. On the other hand, macrophages have a diameter of 10 μm to a maximum of 20 μm and practically have to slide through these small spaces towards the intruding foreign bodies.
To make this path easier for them, cytokines around a foreign body cause local edema that widens the spaces between collagen fibers. This is similar to the situation with injected toxins or venom from bees or snakes, where severe edema on the one hand dilutes the venom, but at the same time facilitates the migration of macrophages to phagocytose and clean up possible cellular debris. The first step of the body’s defense mechanisms is the formation of edema, to allow the defense cells to have a quick path to the incident site. In the case of injectables, what causes problems are not bacteria, toxins or viruses, but microspheres of 40 μm in diameter. Due to their size, they cannot be phagocytosed and transported, but will be enveloped by macrophages and giant cells until they are absorbed, or fixed as PMMA in the tissue for the rest of the patient’s life. As the lifespan of macrophages reaches only a few weeks or months, they must be replaced repeatedly. For this exchange of invading cells and for the transport of cellular debris, the body maintains a chronic edema ready. In the case of subdermal injections of absorbable or non- absorbable microspheres, this edema remains until the end of absorption or until the end of the patient’s life and reaches the structures of the dermis, smoothing superficial wrinkles (KIM, 2019). Collagen is contained in many ointments, however, to date, no molecule has penetrated the skin by this means. The same goes for hyaluronic acids (HA): our body contains about 15 g of HA, mostly in the skin, but millions of women use it in creams or serums, hoping that it will help their skin absorb water, even compressing it. An exception is inflamed skin, whose swollen epidermal cells give way and allow certain antibiotics, cortisone, and non-steroidal anti-inflammatory drugs (NSAIDs) to pass through their intercellular spaces. Our intact skin barrier only allows about 20 or so tricky molecules – like DMSO, estrogens, or nicotine – to permeate, but they disappear in the first lymphatic vessel they hit (MORTAZAVI; MOGHIMI, 2022).
CONCLUSIONS
All particulate injectables stimulate a foreign body reaction: Radiesse® – which is the one with less predominant degradation by osteoclastic hydrolases; Sculptra® – an average response; and Ellansé® – the strongest, with all microspheres being swallowed by foreign body giant cells (KIM, 2019).
All three manufacturers and all users claim that their product is a collagen stimulator, but only PMMA permanent injectables such as Biosimetric®, Linnea Safe®, Artecoll and Bellafill have histologically shown for 40 years that the body’s initial reaction strange settles down within six months and gives way to a stable encapsulation of all microspheres (LEMPERLE et al., 1991; LEMPERLE, 2022). All manufacturers of temporary fillers and their users claim that their product is a collagen stimulator, but only Ellansé® stimulates fibroblasts to secrete mature collagen fibers in their four-year degradation period. In contrast, permanent PMMA microspheres in Biosimetric, Linnea Safe, Artecoll and Bellafill have histologically shown for 40 years that the initial foreign body reaction subsides within six months and gives way to macrophages, for stable encapsulation and fixation with mature collagen fibers (LEMPERLE et al., 1991; LEMPERLE, 2022). Therefore, PMMA microspheres are the only real collagen stimulators (Figures 9.14 and 9.15), even though their manufacturers and users have never claimed this fact.
The hypothesis of neocollagenesis, described and presented a hundred times after Sculptra® and Radiesse® injections, is based on supposed “conventional wisdom” and “positive thinking”, to calm doctors and patients. This was a hypothesis from the beginning because it sounded so much better than the scientific truth of a foreign body reaction. Finally, Kim (2020) presented proof that PLC microspheres also stimulate collagen synthesis after four years, when the degradation of PLC microspheres by giant cells is ending. Just as the shift from foreign body reaction to biostimulation has occurred, the most recent fill-in literature has to avoid the expression foreign body granuloma and replace it with late-onset inflammatory response (LOIR). Similarly, some journals prohibit the word complication and ask to replace it with adverse events (AEs). Unfortunately, even in scientific articles, we are forced to erase reality and discard many facts as old fake news.
REFERENCES
Blanco Souza TA, Colomé LM, Bender EA, Lemperle G. Brazilian consensus recommendation on the use of polymethylmethacrylate filler in facial and corporal aesthetics. Aesthetic Plast Surg. 2018; 42:1244-1251.
Bravo BF, de Menezes Penedo LB, de Melo Carvalho R, Miot HA, Elias MC. Improvement of facial skin laxity by a combined technique with hyaluronic acid and calcium hydroxylapatite fillers: A clinical and ultrasonography analysis. J Drugs Dermatol. 2022; 21:102-106.
Buzzaccarini G, Laganà AS, Borin M et al. Los Deline copolyamide filler for breast and buttock augmentation. The position statement of the Italian Aesthetic Medicine Association (AMEI) J Plast Reconstr Aesthet Surg. 2022 June 20; S1748-6815(22)00371-0.
Cavallini M, Papagni M, Ryder TJ, Patalano M. Skin quality improvement with VYC-12, a new injectable hyaluronic acid: Objective results using digital analysis. DermatolSurg. 2019; 45:1598-1604. Chacur R, Sampaio Menezes H, Maria Bordin da Silva, et al. Gluteal augmentation with polymethylmethacrylate: A 10-year cohort study. Plast Reconstr Surg Glob Open. 2019; 31;7:e2193. Christen MO, Verceri F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin Cosmet Investig Dermatol. 2020; 13:31-48.
Christen MO. Collagen stimulators in body applications: A review focused on poly-l-lactic acid (PLLA). Clin Cosmet Investig Dermatol. 2022; 15:997-1019.
Cohen JL, Sadick NS, Kirby MT, et al. Development and validation clinician and patient reported photonumeric scales to assess buttocks cellulite severity. Dermatol Surg. 2020; 46:1628-1635.
Davis DS, Boen M, Fabi SG. Cellulite: Patient Selection and Combination Treatments for Optimal Results-A Review and Our Experience. Dermatol Surg. 2019 Sep;45(9):1171-1184.
De Melo F, Nicolau P, Piovano L, et al. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin Cosmet Investig Dermatol. 2017; 10:431-440.
Durairaj KK, Devgan L, Lee Bs A, et al. Poly-l-lactic acid for gluteal augmentation found to be safe and effective in retrospective clinical review of 60 Patients. Dermatol Surg. 2020; 46 Suppl 1:S46-S5.
Goldberg D, Guana A, Volk A, Daro-Kaftan E. Single-arm study for the characterization of human tissue response to injectable poly-L-lactic acid. Dermatol Surg. 2013; 39:915-922.
Haddad S, Galadari H, Patil A, Goldust M, Al Salam S, Guida S Evaluation of the biostimulatory effects and the level of neocollagenesis of dermal fillers: a review. Int J Dermatol. 2022 Apr 29. doi: 10.1111/ijd.16229.
Handler MZ, Goldberg DJ. Neocollagenesis. In: Goldberg DJ (ed): Dermal Fillers. Aesthet Dermatol. Basel, Karger, 2018, vol 4, p.27-35. Hexsel DM, Mazzuco R. Subcision: a treatment for cellulite. Int J Dermatol. 2000; 39(7):539-544.
Kaminer MS, Casabona G, Peeters W, et al. Validated assessment scales for skin laxity on the posterior thighs, buttocks, anterior thighs, and knees in female patients. Dermatol Surg. 2019; 45(Suppl.1):S12-S21. Kim J. Isovolemic Degradation of Polycaprolactone Particles and Calculation of Their Original Size from Human Biop- sy. Plast Reconstr Surg Glob Open 2020; 8:e2866.
Kim JA, Van Abel D. Neocollagenesis in human tissue inject- ed with a polycaprolactone-based dermal filler. J Cosmet Laser Ther. 2015; 17:99-101.
Kim JS. Changes in dermal thickness in biopsy study of histologic findings after a single injection of polycaprolactone-based filler into the dermis. Aesthet Surg J. 2019; 39(12): NP484–NP494.
Kruglikov IL. Neocollagenesis in non-invasive aesthetic treatments. J Cosmet Dermatol Sci Appl. 2013; 3:1A001. Lacombe V. Sculptra®: a stimulatory filler. Facial Plast Surg. 2009; 25:95-99.
Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM: Foreign body granulomas after all injectable dermal fillers. Part 1: Possible causes. Plast Reconstr Surg. 2009; 123:1842-63.
Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2020; 44:1348-1360.
Lemperle G, Ott H, Charrier U, Hecker J, Lemperle M. PMMA microspheres for intradermal implantation: Part I. Animal research. Ann Plast Surg. 1991; 26:57-63.
Lemperle G. Human histology of a new bulking agent containing PMMA-microspheres against gastric reflux and incontinence. Int J Innov Res Med Sci (IJIRMS) 2022; 7:177-184.
Mortazavi SM, Moghimi HR. Skin permeability, a dismissed necessity for anti-wrinkle peptide performance. Int J Cos-
met Sci. 2022; 44:232-248. Ray S, Ta HT. Investigating the effect of biomaterials such as poly-(l-lactic Biomater. 2020;11,51 (p.1-9).
Ronan SJ, Eaton L, Lehman A, Erickson CP. Histologic characterization of polymethylmethacrylate dermal filler biostimulatory properties in human skin Dermatol Surg. 2019; 45:1580-1584.
Serra MS, Gonçalves LZ, Ramos-E-Silva M. Soft tissue augmentation with PMMA-microspheres for the treatment of HIV-associated buttock lipodystrophy. Int J STD AIDS. 2014 May 22. pii: 0956462414536878.
Trindade de Almeida A, Figueredo V, Gonzaga da Cunha AL, et al. Consensus recommendations for the use of hy- perdiluted calcium hydroxyapatite (Radiesse®) as a face and body biostimulatory agent. Plast Reconstr Surg Glob Open. 2019; 7:e2160. Wang F, Garza LA, Kang S, et al: In vivo stimulation of de novo collagen production caused by cross-linked hyal- uronic acid dermal filler injections in photodamaged human skin. Arch Dermatol 2007; 143:155-163.
Young VL, Di Bernardo BE. Comparison of cellulite severity scales and imaging methods. Aesthet Surg J. 2021; 41(6):NP521–NP537. Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and décolletage: A Pilot Study. J Drugs Dermatol. 2017; 16:68-74.
